      |

AI in small ruminants is more difficult than in cattle because of the small animal size and the complex anatomy of the cervical canal - making insemination into the uterus difficult. Sheep are the most difficult, goats are the easiest and deer are between the others in difficulty.
The success of an AI program depends on many factors:
| whether fresh or frozen semen is used, | |
| the number of times inseminated during heat, | |
| the time of insemination, | |
| the method and location (vagina, cervix or uterus) of insemination, | |
| the quality and amount of the semen inseminated (number of live sperm cells), | |
| the handling of the semen (fresh or frozen) preparing for the AI, | |
| the management of the animals to be inseminated. |
FRESH SEMEN
Semen is collected and the sample is "extended" with products that increase the volume (so more animals can be inseminated). The extender also provides an energy source to keep it alive longer, and buffers that protect it from cooling damage. Semen can be collected and extended with reasonable fertility expected for the first 6-8 hours. Large doses of the extended -fresh semen (300+ million sperm) can be simply deposited into the vagina with reasonable success - pregnancy rates averaging 50% or better. Success is improved, and doses can be reduced, if the fresh semen is introduced into the cervix, or even better, into the uterus using trans cervical or laparoscopic methods. There are many examples of successful fresh semen AI programs. Because the results of fresh-semen AI are acceptable, the challenge for research is to extend the life of the semen to enable insemination to be done beyond the day of collection.
FROZEN SEMEN
The process of freezing and thawing semen damages the many of the sperm cells which causes reduced fertility. . Results are affected by the numbers of sperm that survived the freezing and thawing. Frozen semen rarely produces fertility as good as fresh semen. To get best pregnancy rates, frozen semen must be deposited into the uterus. The challenge for small ruminants is that the cervix of the sheep, goats and deer is not as insemination-friendly as it is in cattle or even swine. Until recently, it could not be penetrated at all for AI using practical systems.
Approaches used for frozen semen AI:
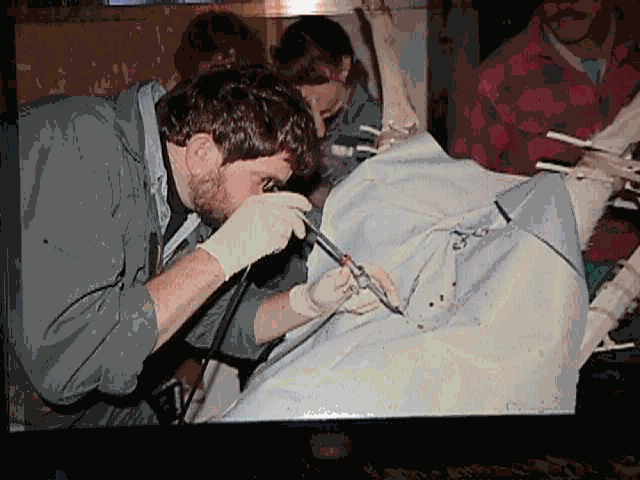
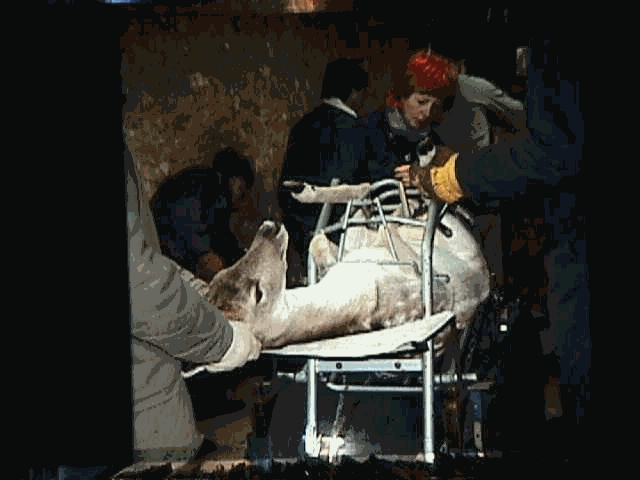
Laparoscopic AI uses a laparoscopic-surgical approach to injecting the semen through punctures made in the abdomen directly into the uterus. Animals are given a sedative/analgesic and undergo preparation as required for any minor surgery. Results are good but the procedure is difficult and costs are relatively high. Millions of sheep are laparoscopically inseminated annually. Almost all deer that undergo AI are done laparoscopically. It is less often required in goats because cervical and transcervical AI produces acceptable results Laparoscopic AI uses a laparoscopic-surgical approach to injecting the semen through punctures made in the abdomen directly into the uterus. Animals are given a sedative/analgesic and undergo preparation as required for any minor surgery. Results are good but the procedure is difficult and costs are relatively high. Millions of sheep are laparoscopically inseminated annually. Almost all deer that undergo AI are done laparoscopically. It is less often required in goats because cervical and transcervical AI produces acceptable results uses a laparoscopic-surgical approach to injecting the semen through punctures made in the abdomen directly into the uterus. Animals are given a sedative/analgesic and undergo preparation as required for any minor surgery. Results are good but the procedure is difficult and costs are relatively high. Millions of sheep are laparoscopically inseminated annually. Almost all deer that undergo AI are done laparoscopically. It is less often required in goats because cervical and trans cervical AI produces acceptable results Laparoscopic AI uses a laparoscopic-surgical approach to injecting the semen through punctures made in the abdomen directly into the uterus. Animals are given a sedative/analgesic and undergo preparation as required for any minor surgery. Results are good but the procedure is difficult and costs are relatively high. Millions of sheep are laparoscopically inseminated annually. Almost all deer that undergo AI are done laparoscopically. It is less often required in goats because cervical and trans cervical AI produces acceptable results
Cervical AI is used to introduce semen into the cervical canal. Cervical AI is used commonly in goats, particularly because many types of insemination equipment are not designed fro passage through the cervical canal into the uterus. Results are considerably better than depositing the semen into the vagina but far less than by depositing the semen into the uterus. Cervical AI with frozen semen should only be done in sheep when attempts to penetrate the cervix have failed the semen is simply "dumped" at the farthest possible site. Cervical AI is used to introduce semen into the cervical canal. Cervical AI is used commonly in goats, particularly because many types of insemination equipment are not designed fro passage through the cervical canal into the uterus. Results are considerably better than depositing the semen into the vagina but far less than by depositing the semen into the uterus. Cervical AI with frozen semen should only be done in sheep when attempts to penetrate the cervix have failed the semen is simply "dumped" at the farthest possible site.


Trans cervical AI (TAI) techniques have been developed to pass semen through the cervix into the uterus. TAI has been used successfully in goats for many years. An experienced inseminator can inseminate most does with acceptable results - as long as adequate number of live sperm are used. Sheep , because of the more complex cervix, have been much more difficult. A TAI technique has been developed at Guelph ( the Guelph System for Trans cervical AI) as an alternative to the laparoscopic technique. Ewes are positioned on their back and insemination equipment is manipulated through the cervix. Unfortunately, not all ewes can be penetrated successfully and pregnancy rates are less than with the laparoscope. An experienced inseminator can normally penetrate 75 % of ewes. The numbers that can be penetrated are higher with ewes on accelerated breeding program or having had multiple lambings. Of those penetrated, in well managed program about 40 to 60 percent lamb. So often only 30 % of those attempted lamb. Still, the system can be managed by trained producers and the success is good enough to introduce new, known health-status genetics into a flock at low cost. A similar system is used with success in anesthetized Fallow and White Tailed deer. The Guelph System works very well in goats, in which almost 100% can be penetrated and in inseminated into the uterus. Goats do not need, in fact would not accept, being positioned on their backs. Goats are simply bred standing. Trans cervical AI (TAI) techniques have been developed to pass semen through the cervix into the uterus. TAI has been used successfully in goats for many years. An experienced inseminator can inseminate most does with acceptable results - as long as adequate number of live sperm are used. Sheep , because of the more complex cervix, have been much more difficult. A TAI technique has been developed at Guelph ( the Guelph System for Trans cervical AI) as an alternative to the laparoscopic technique. Ewes are positioned on their back and insemination equipment is manipulated through the cervix. Unfortunately, not all ewes can be penetrated successfully and pregnancy rates are less than with the laparoscope. An experienced inseminator can normally penetrate 75 % of ewes. The numbers that can be penetrated are higher with ewes on accelerated breeding program or having had multiple lambings. Of those penetrated, in well managed program about 40 to 60 percent lamb. So often only 30 % of those attempted lamb. Still, the system can be managed by trained producers and the success is good enough to introduce new, known health-status genetics into a flock at low cost. A similar system is used with success in anesthetized Fallow and White Tailed deer. The Guelph System works very well in goats, in which almost 100% can be penetrated and in inseminated into the uterus. Goats do not need, in fact would not accept, being positioned on their backs. Goats are simply bred standing.
SEMEN QUALITY AND QUANTITY
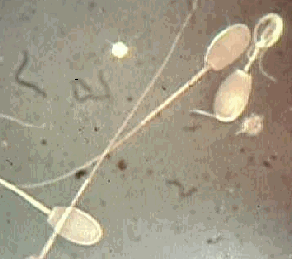
There is a considerable amount of difference between males in the quality of their semen. As well, individual males do not produce semen of consistent quality - one day can be better than the next. As a result, not all semen available for AI is of equal fertility. Especially for fresh AI when the semen must be used from that day's collection. Methods used to evaluate, particularly in the field (simple numbers and motility) are very crude methods of evaluation. In some cases, two samples, appearing to be similar using crude evaluation methods may have different success rates using AI. In many cases, the poor quality semen can be compensated for (at least partially) by increasing the numbers of sperm cells inseminated - trying to get more normal cells. In all cases, using more sperm cells are better.
SEMEN HANDLING
Semen is very sensitive to changes in temperature. High temperatures increases the metabolic rate and consumes sperm life. Chilling can damage the sperm.
Fresh semen is prepared with an extender that provides an energy source and protection against cooling. If chilled slowly to refrigeration temperature it must then be held at refrigeration temperature (4-5 C) until just prior to inseminating.
Frozen semen is thawed according to specific protocols provided by those freezing the semen. Typically, each straw is warmed according to protocol ( a typical protocol is to placed the semen straw in 35C water for 30 seconds) and used immediately without time for further temperature change. A severely cold or hot room temperature will affect results.
ANIMAL PREPARATION
In most AI programs adequate amounts of good quality semen are inseminated using the good technique. In such cases, variation in success is often due to animal selection, preparation and handling.
The goal of an AI program is to deposit semen at a chosen time in advance of when the animal ovulates - normally in the last 1/3 of the heat period. Fresh semen placed into the vagina should be deposited about 8 hours in advance of ovulation. Frozen semen has a shorter life and should be deposited closer to ovulation time - ideally about 4 hours before. The problem is that all animals in the group won't ovulate at the same time, even in synchronized heat programs. Animals are brought into synchronized estrus using progestagen hormones in vaginal pessaries and pregnant mares serum gonadotropin (PMSG). By following directions most will come into heat at about the same time. Still, there is considerable variation in the time of ovulation amounts animals in the group. Some will ovulate at less than 60 hour from pessary removal, most about 60-65 hours and some not until over 70 hours. So the inseminator is blind to the ideal time for each animal. Still, there are things we can do to further tighten the variation in ovulation. Dairy producers need to insure adequate drug withdrawal times following synchronization programs. A natural progesterone device (CIDR) should be considered.

By introducing a teaser (teasing with an intact male works reasonably well but not as good as actually letting the teaser breed ) at first signs of heat, the ovulations in those that would have ovulated later than others are moved ahead. Similarly, by injecting GnRH hormone about 24 hours after pessary removal, it is reported that most will ovulate sooner.
Sheep should be bred with fresh semen about 48 to 55 hours after pessary removal. With frozen semen, TAI is done at about 54 hours and laparoscopic AI at about 56 hours. Deer are laparoscopically AI's at over 65 hours from progesterone removal.
Goats, particularly dairy goats, can often be bred in natural heats. Single inseminations are done at about 36 hours after heat begins. Best program is to breed at 12 and 24 hours after first signs of heat.Typical Results From AI
| SEMEN TYPE |
DOSE (millions of sperm cells - 50% live) |
METHOD/ LOCATION |
TYPICAL SUCCESS REPORTED % |
RANGE IN SUCCESS % reported |
| FRESH SEMEN | 300 + | VAGINA | 50% | 40-65 |
| 150 | TRANS CERVICAL | 40 %of those penetrated | 50-70 | |
| 50 | LAPAROSCOPIC | 70 % | 60-90 | |
| FROZEN SEMEN | 300+ | VAGINA | 10% | 0-30 |
| 100+ | CERVIX (goats) | 35% | 25-50 | |
| 75-150 | TRANS CERVICAL | 40- 50% of those penetrated | 30-60 | |
| 25-60 | LAPAROSCOPIC | 65% | 50-90 |
Results for laparoscopic AI in deer are often better than those achieved in sheep and goats
TYPICAL SHEEP PROGRAM - PROGRAMS MAY VARY ACCORDING TO CIRCUMSTANCES
| DAY 1 | Day 8 | DAY 10 | DAY 11 | DAY 12 |
| Pessary in | Administer Prostaglandin dose | Pessary out PMSG (300-500 IU) |
Teaser in or GNRH (50 ugm) |
Breeding at fixed
time from pessary removal - 54 hours frozen semen or 50-52 hours fresh semen |
TYPICAL GOAT PROGRAM
| DAY 1 | Day 8 | DAY 10 | DAY 11 | DAY 12 |
| Pessary in | Inject Prostaglandin | Pessary out
plus give PMSG (300-500 IU) |
Tease or GnRH (50 ugm) |
Breed at 24 hours
after first heat or 12 hours after first heat and 12 to 24 hours later Breed when mucous turns cloudy Breeding at fixed time from pessary removal - 54 hours frozen semen or 50-52 hours fresh semen |
DRUGS USED
| DRUGS |
TRADE NAME |
SOURCE |
DOSAGE |
| VAGINAL PROGESTERONE | VERAMIX CIDR (natural progesterone) |
UpJohn Co Vetropharm |
|
| PROSTAGLANDIN |
Estrumate Lutalyse |
Schering Co Upjohn Co |
125 ugm 8 mg |
| PMSG |
Equinex Stimukron Folligon |
Ayerst PVU Intervet |
500iu |
| GNRH | Factrel Cystorelin |
Ayerst CEVA |
50 ugm |
MANAGEMENT FACTORS
| SEASON Season has a big affect on AI. Ewes/does must be brought into an a hormonally-produced heat. Not all respond well. Greater variation occurs between animals. Many animals are not as well conditioned. Light control program are very useful to reduce seasonal affects. These work better in goats than ewes. Many producers doe not understand the principles of light control programs. | |
| BCS Ewes/does must be in good body condition, particularly during winter breedings. High lactation is negative to AI. | |
| DIET Diet is known to have at least two specified effects on reproduction. High protein diets reduce the blood levels of progesterone (hormone required for pregnancy ) causing early death of the embryo. Diets high in legumes, particularly lush new growth, but hays as well, also can contain estrogens that affect sperm and ovulation. Flushing, used frequently in sheep, is only a benefit in over worked or under conditioned animals. And then it is primarily grain (increased energy) that is needed. The flushing should stop following the AI. Lactating goats must not be on a negative energy balance at AI. | |
| STRESS Stress will delay or even prevent ovulation. Animals should not be dewormed, shorn, etc. near the time of AI. Handling at AI must be done with care. | |
| PMSG PMSG must be kept refrigerated from the time of purchase. Once reconstituted, it is to be used immediately. Unused portions can be frozen but must be used within a few months and must not be refrozen. PMSG is to be injected in muscle. | |
| VAGINAL PESSARIES Vaginal pessaries must be kept in a sealed container sealed prior to use. They must be inserted cleanly and correctly. Dirty pessaries can introduce low grade vaginal infections that prevent pregnancy. | |
| TIMING The program is designed to inseminate at the time most females are near the time of ovulation. Follow directions closely on the timing of pessary removal and drug administration. | |
| TEASER ANIMAL A teaser male introduced at 24 hours from pessary removal does two thing. It tightens up the spread of timing of ovulation between females in the group and it identifies those first in heat. Those should be the first inseminated. | |
| GNRH GNRH is administered to reduce the spread in ovulation timing closer to the time of AI - much like the teaser male. | |
| Location of insemination. Insemination on farm can reduce stress. However, for laparoscopic AI, on farm insemination greatly increases costs and does not seem to affect results seriously. Cost benefit needs to be considered. |
CLIENT RESPONSIBILITIES FOR AI
| Follow the recommended schedule closely | |
| Insert the vaginal pessary in a correct and clean fashion. | |
| Avoid high legume hay or pasture flushing prior to or following the AI. | |
| If flushing, use properly balanced energy sources (grains) | |
| Have ewes in good body condition . | |
| Have ewes in dry fleece. | |
| Have a clean, warm, draft free environment for the AI. | |
| Handling facilities must be suitable and located close to the site for AI. | |
| The client is to have at least two individuals capable of all animal handling and lifting. | |
| For laparoscopic AI, have ewes in short fleece, have animals off all food and water for 18 hours in advance. | |
| If done at home, have adequate facilities for working - ask for details. | |
Do not being animals to SRGenetics facilities with ectoparasites, foot rot or any infectious diseases of concern. |